Both methodologies are made in human beings who voluntarily participate, they consist in the determination of the minimum erythema dose on the unprotected skin (no product applied) and on protected skin (product applied). The minimum erythema dose correspond to the minimum radiation dose necessary to produce the first visible erythemal reaction with clearly defined edges. The erythema reaction is a biological reaction mainly caused by UVB radiation, and the main visible sign is redness and burning sensation on the skin (sunburn).
The ration between the minimum erythema dose on protected skin and unprotected skin corresponds to the SPF valued, which represents the extra period of time that the skin can be exposed to sunlight with protection granted by the sunscreen. For example, if someone takes 10 minutes of sunlight exposure to get a sunburn, when this individual uses a sunscreen SPF 30 the exposure time increases to 300 minutes of sunlight before getting a sunburn. Meaning, the exposure time increases 30 times.
CLAIM SUPPORT
Adjustments can be made to support immediate protection claims, in a study in which the product is applied to dry skin and it is exposed to irradiation immediately after application. We can also offer evaluation methods of photoprotection provided by the product after application to wet skin.
Lab focusing in STATIC SPF, where more than 300 TESTS are conducted every year.
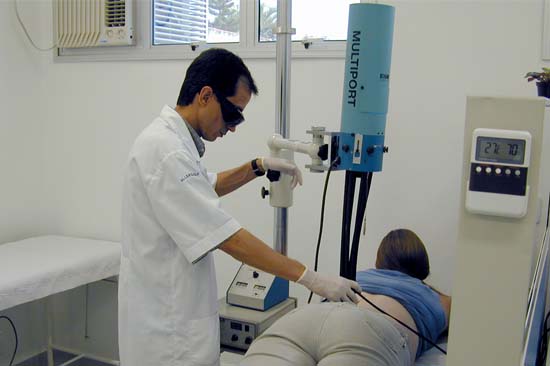
Allergisa owns a total of 8 solar simulators Multiport from Solar Light, which are calibrated on regular basis by the internal optical lab - member of the Brazilian Calibration Network (RBC) and accredited by Cgcre under the CAL 0549 - in order to confirm the compliance of all requirements of the reference standards to assure the safety of the study subjects and consistent results.
In addition to trained technicians, pieces of equipment kept compliant and enhanced infrastructure for photoprotection studies, Allergisa joins on regular basis proficiency tests (interlaboratorial), in which results obtained from labs all over the world are compared to each other, presenting satisfactory results.

 Portuguese
Portuguese
 Español
Español